The Rate Constant For A First Order Reaction Becomes Six Times When Th

The Rate Constant For A First Order Reaction Becomes Six о The rate constant of a first order reaction becomes 5 times when the temperature is raised from 350 k to 400 k. calculate the activation energy for the reaction. (gas reactant r = 8.314 j k − 1 m o l − 1 ). The rate of a reaction becomes 4 times when temperature is raised from 293 k to 313 k. the activation energy for such reaction would be asked jul 14, 2019 in chemistry by kumari prachi ( 83.3k points).
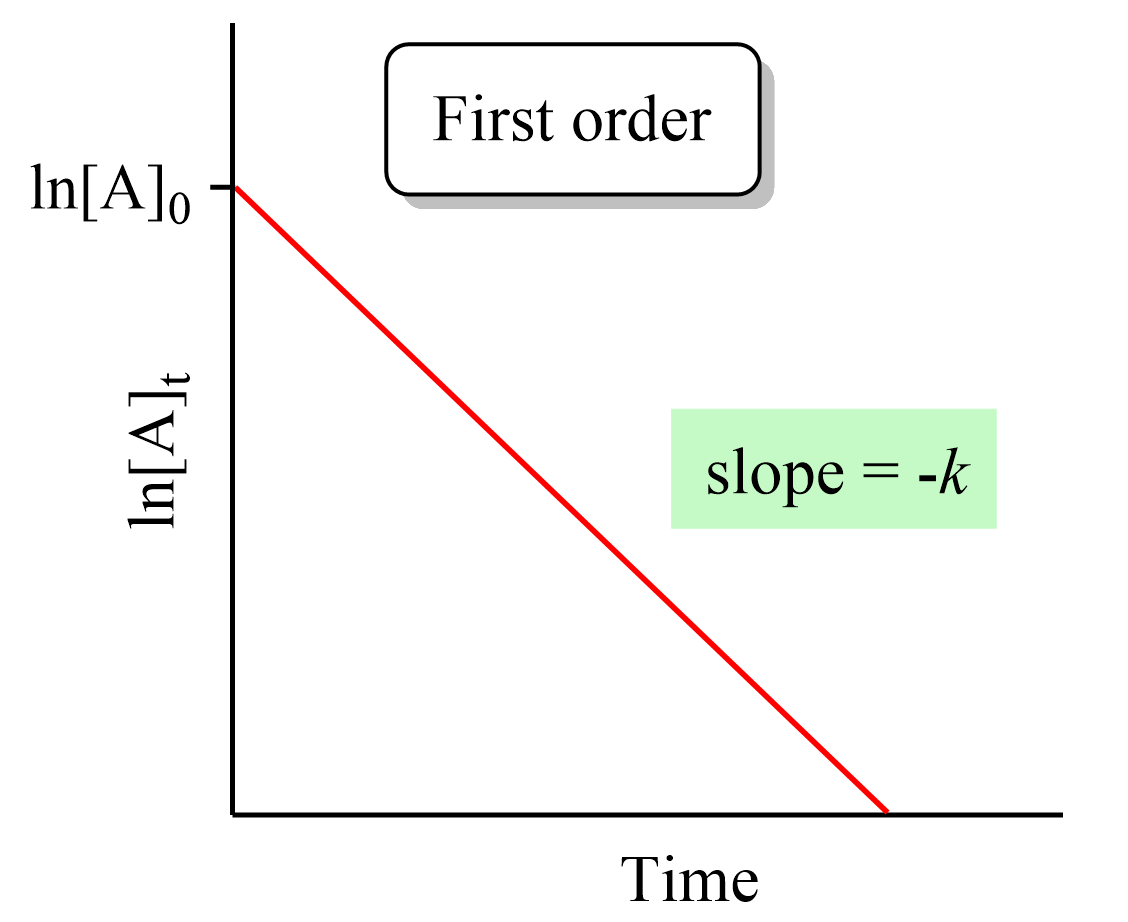
First Order Reactions Chemistry Steps The rate constant of a first order reaction is 6 times when the temperature is increased from 350 k to 410 k. the energy of activation for the reaction is (i n k j m o l − 1). write it in 10x form. ( write your answer to nearest integer). The rate constant for a first order reaction becomes six times when the temperature is raised from 350 k to 400 k. calculate activation energy for the reacti cbse exam, class 12. Answer for the rate constant of a first order reaction becomes 6 times when the temperature is increased from 350 k to 410 k. calculate the energy of activation for the reaction.(r = 8.314 jk 1 mol 1) ktunc6wkk. The rate constant for a first order reaction becomes six times when the temperature is raised from 350 k to 400 k. calculate the activation energy for the reaction ( r = 8.314 jk 1 mol 1 ) q. the rate constant for a first order reaction becomes six times when the temperature is raised from 350 k to 400 k. calculate the activation energy for.
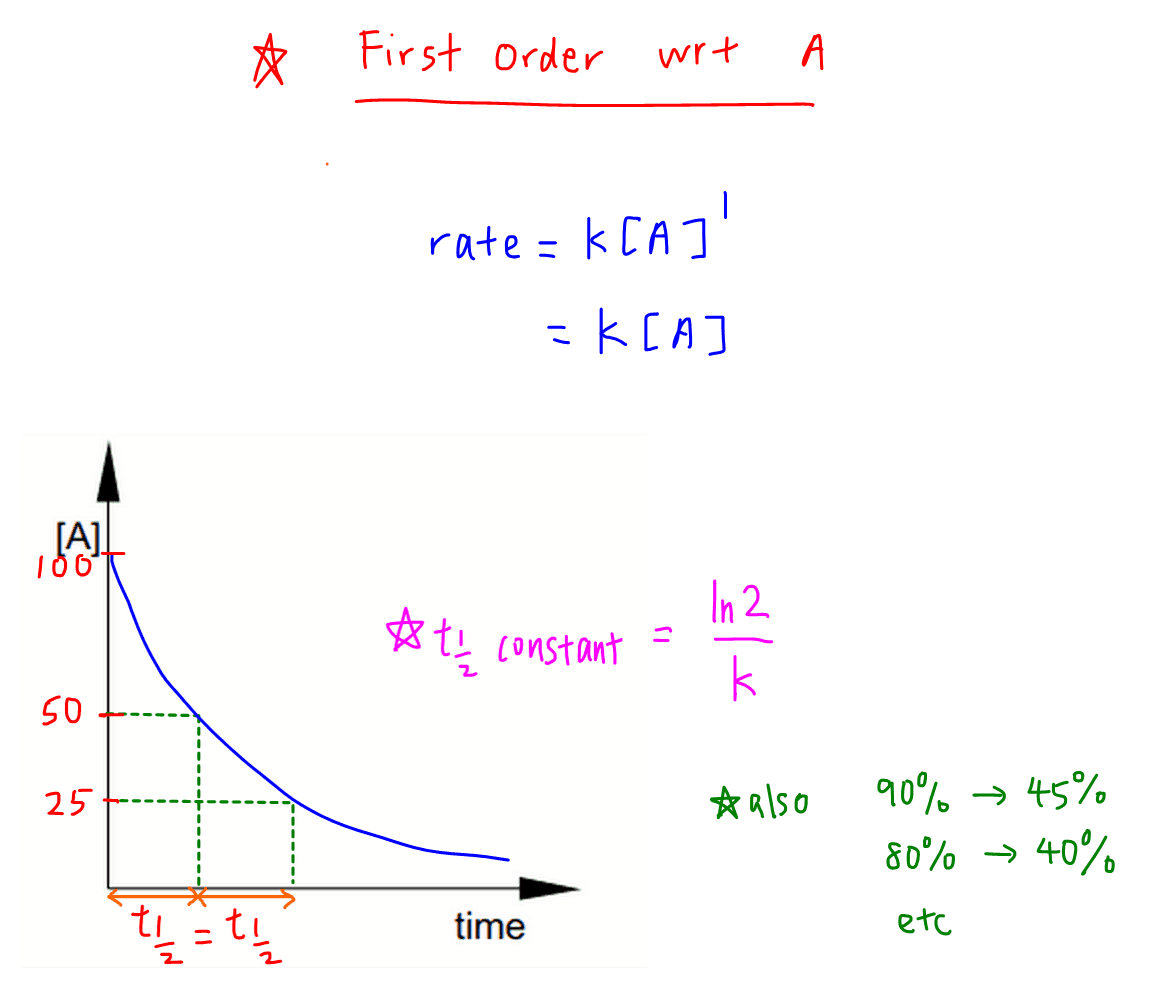
Rate Equation And Order Of Reaction Answer for the rate constant of a first order reaction becomes 6 times when the temperature is increased from 350 k to 410 k. calculate the energy of activation for the reaction.(r = 8.314 jk 1 mol 1) ktunc6wkk. The rate constant for a first order reaction becomes six times when the temperature is raised from 350 k to 400 k. calculate the activation energy for the reaction ( r = 8.314 jk 1 mol 1 ) q. the rate constant for a first order reaction becomes six times when the temperature is raised from 350 k to 400 k. calculate the activation energy for. The rate constant for a first order reaction becomes six times when the temperature is raised from $350{\text{ k}}$ to $400{\text{ k}}$. consider ${t 1} = 350{\text{ k}}$ and ${t 2} = 400{\text{ k}}$. 2.75 × 10 −7. answer: first order; k = 2.2 × 10 −5 s −1. we can also use the integrated rate law to determine the reaction rate for the hydrolysis of cisplatin. to do this, we examine the change in the concentration of the reactant or the product as a function of time at a single initial cisplatin concentration.

T1 2 For A First Order Reaction Is 6 93 S The Value Of Rate Constan The rate constant for a first order reaction becomes six times when the temperature is raised from $350{\text{ k}}$ to $400{\text{ k}}$. consider ${t 1} = 350{\text{ k}}$ and ${t 2} = 400{\text{ k}}$. 2.75 × 10 −7. answer: first order; k = 2.2 × 10 −5 s −1. we can also use the integrated rate law to determine the reaction rate for the hydrolysis of cisplatin. to do this, we examine the change in the concentration of the reactant or the product as a function of time at a single initial cisplatin concentration.

Comments are closed.