Using The Figure Shown Below As Reference Draw The Resonance
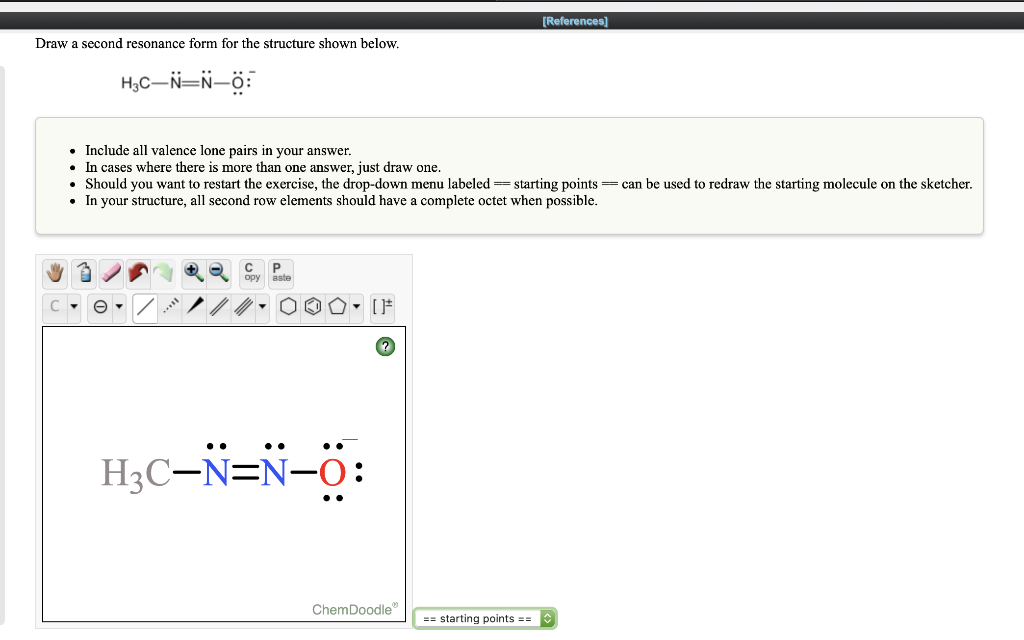
Solved References Draw A Second Resonance Form For The Chegg Exercise 2.6.6: draw the major resonance contributor for each of the anions below. c) fill in the blanks: the conjugated pi system in part (a) is composed of 2p orbitals containing delocalized pi electrons. exercise 2.6.7: the figure below shows how the negative formal charge on the oxygen can be delocalized to the carbon indicated. A) draw three additional resonance contributors for the carbocation below. include in your figure the appropriate curved arrows showing how one contributor is converted to the next. b) fill in the blanks: the conjugated pi system in this carbocation is composed of 2p orbitals sharing delocalized pi electrons.

Solved References A Draw The Additional Resonance Chegg Prof. steven farmer (sonoma state university) 2.14.1: drawing resonance forms using pattern recognition is shared under a cc by nc sa 4.0 license and was authored, remixed, and or curated by libretexts. resonance structures are used when one lewis structure for a single molecule cannot fully describe the bonding that takes place between. Resonance and curly arrows. 10th apr 2020. in this article, i will present some guidelines about how to draw and follow curly arrow notation (figure 10.1) when drawing resonance structures (resonance forms). along the way, i will also justify the application of curly arrows by examining the electronic properties of molecules. 1. draw the resonance contributors that correspond to the curved, two electron movement arrows in the resonance expressions below. 2. in each resonance expression, draw curved two electron movement arrows on the left side contributor that shows how we get to the right side contributor. be sure to include formal charges. Figure: resonance and mos. the two major contributing resonance structures of formamide are shown above. below is a rotatable 3d structure showing the delocalized π mo of formamide. when looking at the left resonance structure, you might be tempted to assign sp 3 hybridization to n given its similarity to ammonia (nh 3). however, this is a.

Solved References Draw Both Resonance Structures Of The Chegg 1. draw the resonance contributors that correspond to the curved, two electron movement arrows in the resonance expressions below. 2. in each resonance expression, draw curved two electron movement arrows on the left side contributor that shows how we get to the right side contributor. be sure to include formal charges. Figure: resonance and mos. the two major contributing resonance structures of formamide are shown above. below is a rotatable 3d structure showing the delocalized π mo of formamide. when looking at the left resonance structure, you might be tempted to assign sp 3 hybridization to n given its similarity to ammonia (nh 3). however, this is a. Consider the structure of a carboxylic ester as shown below. at first sight, this might look like ‘half a carbonate’, which would mean that both shown resonance structures are equal, contribute $0.5$ to the overall picture and therefore both bond orders would be $1.5$. however, this is only the case if we are talking about the carboxylate. Step 2: draw curved arrows to show the movement of electrons and resonance structures. to draw the first resonance structure, we will take two electrons out of the double bond shown and shift them.
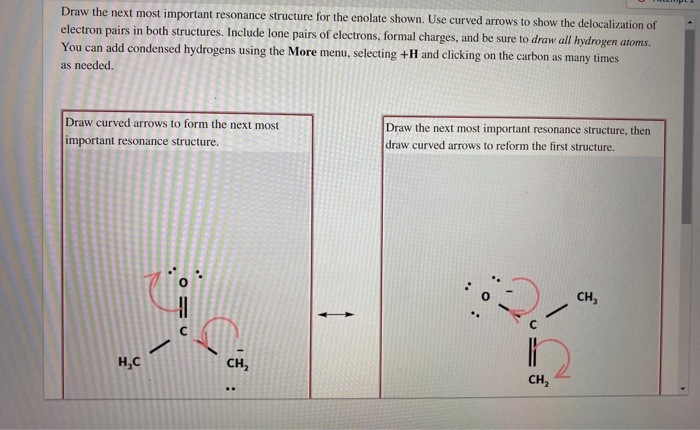
Solved Draw The Next Most Important Resonance Structure For Chegg Consider the structure of a carboxylic ester as shown below. at first sight, this might look like ‘half a carbonate’, which would mean that both shown resonance structures are equal, contribute $0.5$ to the overall picture and therefore both bond orders would be $1.5$. however, this is only the case if we are talking about the carboxylate. Step 2: draw curved arrows to show the movement of electrons and resonance structures. to draw the first resonance structure, we will take two electrons out of the double bond shown and shift them.

Comments are closed.