What Is The Unit Of Rate Constant For First Order Reaction
What Is The Unit Of Rate Constant For First Order Reaction Because the units of the reaction rate are always moles per liter per second, the units of a first order rate constant are reciprocal seconds (s −1). the integrated rate law for a first order reaction can be written in two different ways: one using exponents and one using logarithms. the exponential form is as follows:. From this equation, a general formula for the units of k is obtained which is: k units = m1 n · t 1. where n is the reaction order. for example, let’s say we want to determine the units of the rate constant for third order reactions. n = 3, and therefore, k units = m1 3 · t 1 = m 2 · t 1. if the time is seconds, then the units will be:.
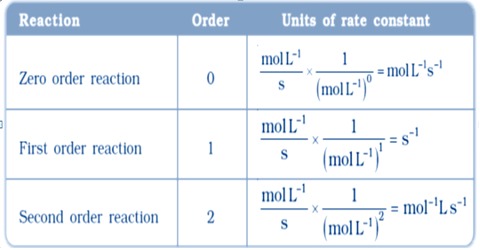
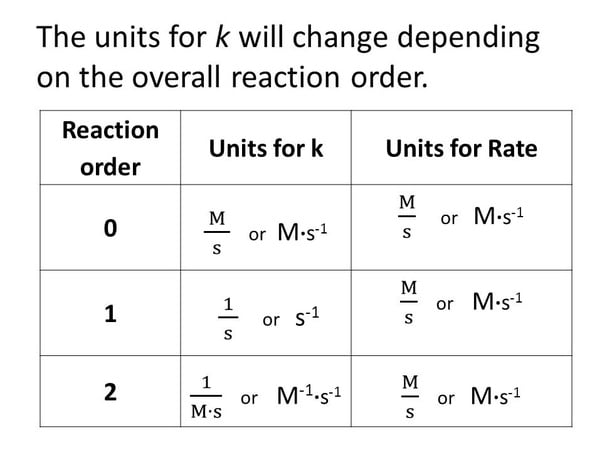
Units Of Rate Constants Qs Study The differential equation describing first order kinetics is given below: rate = − d[a] dt = k[a]1 = k[a] the "rate" is the reaction rate (in units of molar time) and k is the reaction rate coefficient (in units of 1 time). however, the units of k vary for non first order reactions. these differential equations are separable, which simplifies. If m = 1 and n = 1, the overall order of the reaction is second order (m n = 1 1 = 2). the rate law: rate = k[h 2o 2] describes a reaction that is first order in hydrogen peroxide and first order overall. the rate law: rate = k[c 4h 6]2. describes a reaction that is second order in c 4 h 6 and second order overall. The units of the rate constant, k, depend on the overall reaction order. the units of k for a zero order reaction are m s, the units of k for a first order reaction are 1 s, and the units of k for a second order reaction are 1 (m·s). For a third order reaction, the rate constant has units of liter squared per mole squares per second (l 2 ·mol −2 ·s −1) or (m −2 ·s −1) other calculations and simulations for higher order reactions or for dynamic chemical reactions, chemists apply a variety of molecular dynamics simulations using computer software.
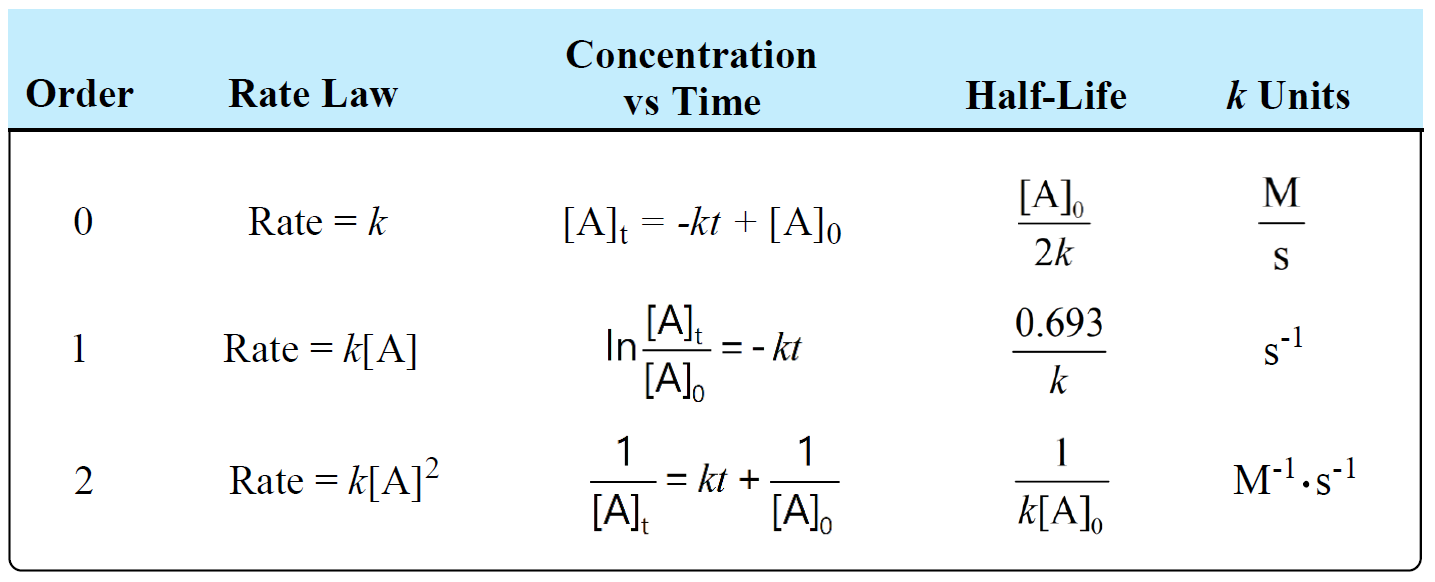
Units Of Rate Constant K Chemistry Steps The units of the rate constant, k, depend on the overall reaction order. the units of k for a zero order reaction are m s, the units of k for a first order reaction are 1 s, and the units of k for a second order reaction are 1 (m·s). For a third order reaction, the rate constant has units of liter squared per mole squares per second (l 2 ·mol −2 ·s −1) or (m −2 ·s −1) other calculations and simulations for higher order reactions or for dynamic chemical reactions, chemists apply a variety of molecular dynamics simulations using computer software. Differential rate law for a first order reaction. a differential rate law can be employed to describe a chemical reaction at a molecular level. the differential rate expression for a first order reaction can be written as: rate = d[a] dt = k[a] 1 = k[a] where, ‘k’ is the rate constant of the first order reaction, whose units are s 1. Differential rate law. the differential rate law gives the derivative of the reactant’s concentration with time. for a first order reaction, it is given as, r = – d [a] dt = k [a] where, r is the reaction rate. [a] is the concentration of the reactant a. k is the rate constant. the term d [a] dt is the derivative of [a] with time.
First Order Reaction Units Differential rate law for a first order reaction. a differential rate law can be employed to describe a chemical reaction at a molecular level. the differential rate expression for a first order reaction can be written as: rate = d[a] dt = k[a] 1 = k[a] where, ‘k’ is the rate constant of the first order reaction, whose units are s 1. Differential rate law. the differential rate law gives the derivative of the reactant’s concentration with time. for a first order reaction, it is given as, r = – d [a] dt = k [a] where, r is the reaction rate. [a] is the concentration of the reactant a. k is the rate constant. the term d [a] dt is the derivative of [a] with time.

Comments are closed.